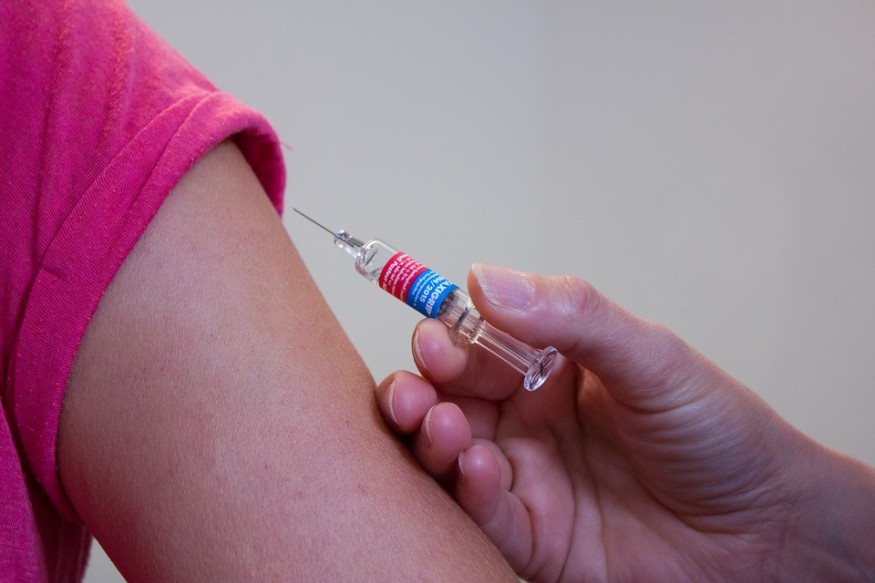
The National Institute of Allergy and Infectious Diseases announced that the first phase of human testing of the experimental vaccine for the new coronavirus started last Monday.
Four adult volunteers received their first doses of a trial vaccine developed through a partnership between the US National Institute of Allergy and Infectious Diseases (NIAID) and Moderna, a biotechnology company based in Cambridge, Massachusetts. Although the phase I trial is an important milestone, the process to test the drug's safety and efficacy is long and tedious. Despite the unprecedented rate at which the vaccine was developed, the vaccine will not be available for at least a year, even if the vaccine would be proven safe and effective.
The study is being conducted at Kaiser Permanente Washington Health Research Institute and will entail testing a range of doses of the vaccine. The participants will receive their first doses over the next 6 weeks. The second dose will be administered 28 days later. The participants' health will be assessed over a 14-month period, mostly requiring the participants to do follow-up visits in person and on the phone. The blood samples will help researchers evaluate the body's immune response to the vaccine on trial.
The first set of tests aims to assess if the vaccine is safe. If proven safe, the study will then determine whether it stimulates the immune system to make antibodies that can stop the virus from replicating and prevent the illness it causes.
Dr. Anthony Fauci, the institute's director, admits that the trail was "launched in record speed". The researchers were able to use their knowledge about related coronaviruses that had caused global outbreaks such as SARS and MERS, contributing to the unprecedented development of a potential vaccine.
Interestingly, Moderna did not need the coronavirus itself to produce its vaccine. The company uses genetic material, the messenger RNA to produce vaccines. A stretch of RNA required for the vaccine was synthesized and embeds it in lipid nanoparticle. Moderna calls the vaccine mRNA-1273.
In fact, the company has nine others in various stages of development. It is the first time that vaccine using the mRNA technology has not reached the market yet. Dr. Barney Graham, the deputy director of the institute's Vaccine Research Center said that the RNA approach can produce vaccines at a fast rate.
Should the trial find that the vaccine is safe, Moderna will ask the help of the Food and Drug Administration to allow them to move to the next phase of testing even before the first stage is finished.
Moderna is purchasing new equipment to be able to produce millions of doses despite neither safety nor efficacy has been approved yet. "humans are suffering, and time is of the essence" Stéphane Bancel, the chief executive of Moderna said.
"Every day matters. We have taken these decisions to take the risk because we believe it is the right thing to do."
As of writing, there is a total of 244,725 cases around the world, with China having no new cases reported.
© 2025 NatureWorldNews.com All rights reserved. Do not reproduce without permission.





